Co-Kneader Skw105 For Shearing,Sjw100 Co-Kneader For Toner,Used In Engineering Plastics,Rubber Mixing Machine JIANGSU XINDA TECH LIMITED , https://www.xindaextruder.com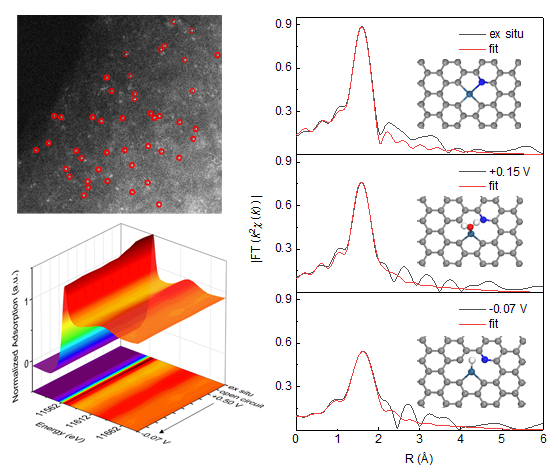
Near-free catalytic kinetics of a single atom discovered by in situ synchrotron radiation
[ Instrumentation Network Instrumentation R & D ] In the field of energy catalysis, it is of great significance for the rational design of catalysts to accurately detect the dynamic changes of the atomic-scale structure of the catalysts in service. In recent decades, scientists' in-depth understanding and controllable construction of nanoscale materials have greatly promoted the development of basic and applied research in catalytic science.
A single atom is a substance that can be formed by one atom. Generally, there are only inert gases (rare gases) because they satisfy eight electrons at the end of each cycle (except the outermost layer of He is a two-electron structure). In addition to rare gases, some elements can form monoatomic molecules, such as atomic hydrogen, under certain conditions. Normally, there are only rare gas elements (only helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), krypton (Rn), irrespective of the 118 element that does not have an aggregated form ( Og), solid non-metals and general non-metals are not monoatomic molecules, but some metal vapors can be considered as monoatomic molecules because the atoms basically exist independently. Metals are directly composed of atoms and are connected to each other by metal bonds.
Among them, monoatomic catalysts have become star materials in the field of energy catalysis in recent years due to their high atom utilization efficiency, excellent reactivity and selectivity. The activity and selectivity of monoatomic catalysts are highly dependent on the local atomic and electronic structure of their metal atoms and their interaction with the support. However, in the actual reaction state, a single-atom catalyst is excited by the external environment such as temperature, electric field, and light, and a structural evolution response occurs. Therefore, in-situ real-time online characterization of this special structure response behavior is important for understanding the nature of single-atom catalysis. And found that the new catalytic phenomenon has important enlightening significance.
Synchrotron X-ray absorption spectroscopy is one of the powerful tools for describing the local space and electronic structure of the catalytic center. It can realize the in-situ detection of solid, liquid and gaseous samples in the actual catalytic environment. Therefore, high-brightness and high-sensitivity advanced synchrotron radiation sources provide an opportunity to study this urgently needed problem.
X-ray absorption spectroscopy (abbreviation: XAS) is a widely used method for obtaining structural information and electronic states of target atomic regions (atomic scales) in gaseous, molecular, and condensate (for example, solid). technology. The X-ray absorption spectrum can be obtained by scanning the X-ray photon energy within the excitation energy range of the target atom's bound electrons. Synchrotron radiation facilities are usually required to provide high intensity and variable wavelength X-ray beams.
Recently, Professor Yao Tao's group at the University of Science and Technology of China developed in situ synchrotron radiation absorption spectroscopy technology and discovered the nearly free evolutionary dynamics of platinum (Pt) monoatomic catalysts in electrocatalytic reduction reactions. Greatly promoted the hydrogen evolution performance of Pt monoatomic catalysts in a wide range of pH electrolytes.
The research team developed synchrotron radiation in situ X-ray absorption spectroscopy technology, selected a Pt monoatomic catalyst with a highly uniform active site as a model catalyst, and monitored the evolution of the Pt monoatomic site under the working condition of electroreduction in real time. It was observed that under a certain reduction voltage, the coordination between Pt atoms and C / N atoms on the carrier decreased, the valence state of Pt decreased, and the electronic structure of Pt was close to a free single atom. Theoretical calculations show that after this dynamic evolution, the d-band structure of the Pt atom is optimized, which promotes the adsorption of water molecules by the active site of the Pt single atom, and facilitates the adsorption and desorption of hydrogen intermediates, which makes the catalyst in Excellent hydrogen evolution performance in a wide range of pH electrolytes. This study successfully used in situ X-ray spectroscopy to accurately characterize the three-dimensional atomic and electronic structures of Pt1 / NC monoatomic catalysts, and also found a universal near-free monoatomic dynamics in the working state of electrocatalytic reduction. Evolution has provided new ideas for the structural design and regulation of energy catalysts in the future.
Source: Encyclopedia, University of Science and Technology of China